Fick's Law of Diffusion
The fundamental equation (one-dimensional) of molecular diffusion is known as Fick's law. It has been derived from the kinetic theory of gases, and can be written for a binary mixture as
JA = –DAB (d CA/dx) (5.8)
where DAB = diffusion coefficient of species A with respect to species B,
JA = molar flux in the X-direction relative to the molar average velocity,
dCA/dx = Concentration gradient in X-direction.
Let us consider a two compartment tank as shown in Fig. 5.1. One compartment contains gas A and the other compartment contains gas B and both the compartments are initially at a uniform pressure and temperature throughout. When the partition between the compartments is removed, the two gases will diffuse through each other until equilibrium is established and the concentration of the gases is uniform throughout the tank.
Fig. 5.1 Diffusion of species A in to species B
Fig 5.2 illustrates the dependence of diffusion on the concentration profile. The concentration of the species A on the left side of the imaginary plane is greater than that on the right side. As such, more molecules will cross the plane per unit time from left to right. This would lead to a net transfer of mass from the region of higher concentration to the region of lower concentration.
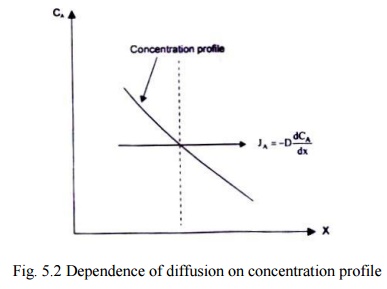
Fig. 5.2 Dependence of diffusion on concentration profile
* This law assumes that fluxes are measured relative to the coordinates that move with the average velocity of the mixture.

